Over the past couple of decades, the significant role of gut-related processes in the development and progression of PD has become increasingly evident.2,3 The involvement of the gastrointestinal system in PD was recognised more than 200 years ago by James Parkinson in his seminal essay on the ‘Shaking Palsy’, where he described symptoms of constipation and a “disordered state of the stomach and bowels”.4 Today, we know that PD gives rise to an array of gastrointestinal symptoms and clinical manifestations and that these can precede the onset of motor symptoms by decades.5,6 Constipation is a classical prodromal feature of PD and, moreover, appears to predict a worse disease trajectory.6-8 Irritable bowel syndrome is common in patients with PD, and people with inflammatory bowel disease have a higher risk of PD than those without.9 Multiple dietary factors have also been associated with increased or decreased risk of PD, which, at least in some cases, may be mediated via the gut.1
The onset and severity of gastrointestinal dysfunction varies between patients, but almost every patient with PD will eventually experience at least one gastrointestinal symptom.5
Almost every patient with PD will eventually experience at least one gastrointestinal symptom.5
The gut-brain axis is a highway for gut-brain communication
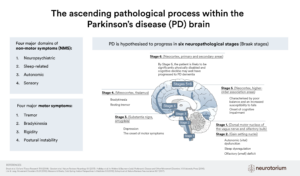
Interest in gut-brain communication in PD was ignited by studies by Braak and colleagues in 2006 who demonstrated alpha-synuclein aggregates (a pathological hallmark of PD) in the brains and in the enteric nervous systems of autopsied individuals with PD, leading the authors to hypothesise that PD may start in the gut, possibly via a pathogen capable of passing through the gastric epithelial lining.10
Studies in animal models have since demonstrated that alpha-synuclein can indeed spread from the gastrointestinal tract to the brain as well as from the brain to the gastrointestinal tract via the vagal nerve.11-13 The bidirectional communication between the central and enteric nervous system has become known as the gut-brain-axis. Key players in this flow of information have been proposed to include gut inflammation (and its association with inflammatory bowel diseases and the LRRK gene), gut hyperpermeability (‘leaky gut’), seeding and propagation of alpha-synuclein in the enteric nervous system, and, more recently, the gut microbiome (Figure 1).1,11
The gut-brain axis allows a bidirectional flow of information between the gut and the brain.1,11-13
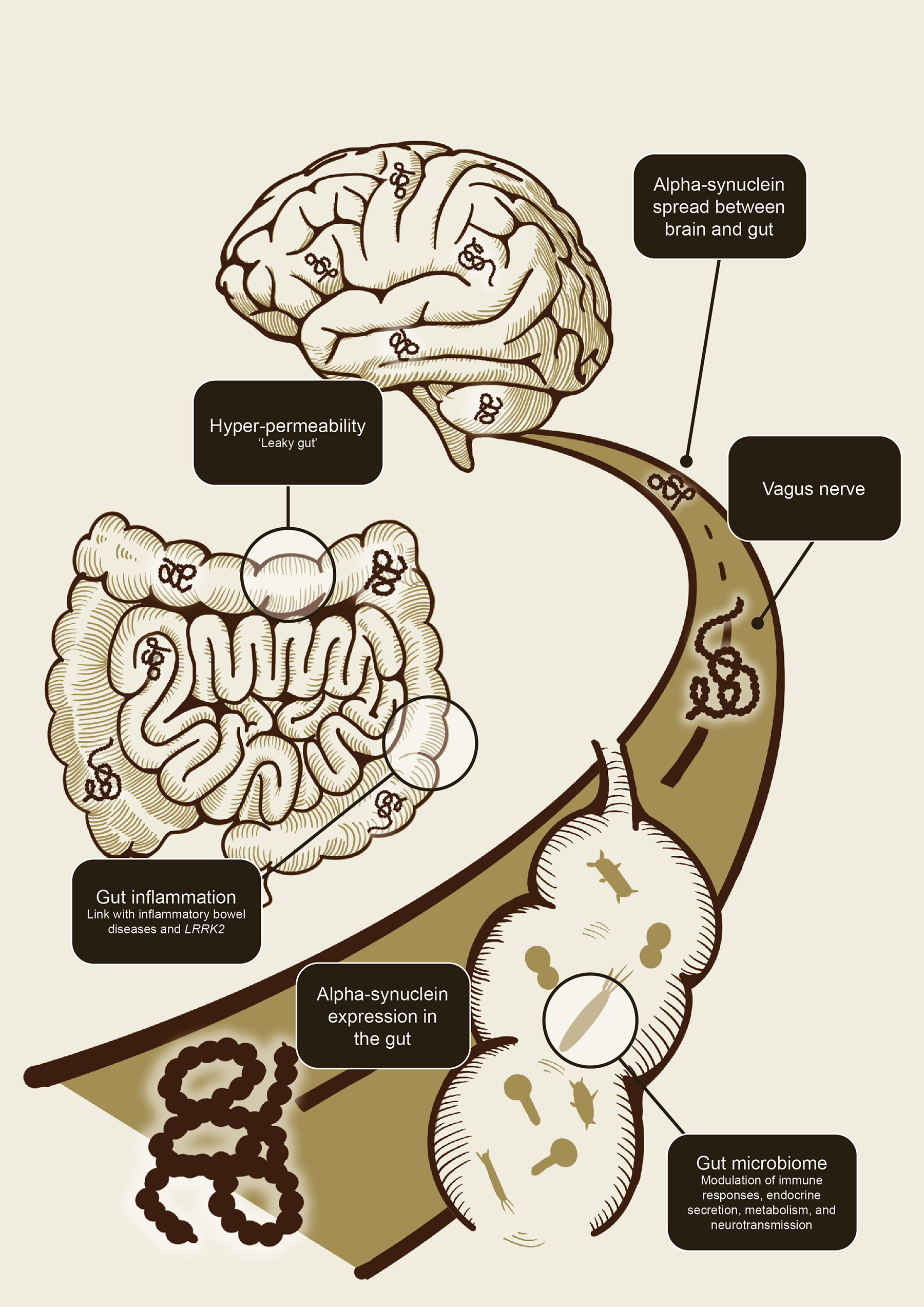
Alpha-synuclein, gut inflammation, and intestinal permeability are key players in the gut-brain axis
It has been hypothesised by some that misfolded aggregates of alpha-synuclein that originate in the enteric nervous system travel to the brain via prion-like, trans-synaptic cell-to-cell transfer, although the mode of transfer as well as the origin of alpha-synuclein are still unclear.11,14 Whether PD starts in the brain or whether it starts in the gut, or if there are both gut-first and brain-first phenotypes of the disease is an area under active investigation.6,15-17
Whether PD starts in the brain or whether it starts in the gut remains undetermined.6,15-17
In other studies, it has been shown that altered intestinal permeability (‘leaky gut’) and gut inflammation also play a role in the pathology of PD in subsets of patients.1,18,19
Interestingly, some types of infections (and subsequent intestinal wall inflammation) have been shown to induce expression of alpha-synuclein in the gastrointestinal tract, which has led to the hypothesis that alpha-synuclein expression may be an immune defence mechanism.11 Chronic pro-inflammatory immune activity is increasingly recognised as a fundamental element of neurogenerative disorders, including PD.19 The recognition of a connection between inflammation and PD has been reinforced by observational studies demonstrating strong links between PD and inflammatory bowel diseases.18,20 In epidemiological and genetic studies, people with inflammatory bowel disease were found to be up to 40% more likely to develop PD compared to those without inflammatory bowel disease, with LRRK2 emerging as a common susceptibility gene for both diseases.18,20
The gut microbiome and its effect on host health
One of the most intriguing discoveries related to this field is the role of the gut microbiome in PD. A huge microbial community known as the microbiota resides on and within the human body. This unseen ecosystem of microbes is located on external and internal surfaces such as the skin, the oral cavity, and particularly, within the gastrointestinal tract.21,22 The gut microbiome is comprised mainly of bacteria, but also includes fungi, viruses, and protozoa, as well as their collective genomes and the environment that they inhabit.23 These microbes act as a vast complex ecosystem in which each species has the potential to exert diverse effects on neighbouring species.24 The most recent estimates suggest that, for the average person, bacterial and human cells are present in roughly equal numbers.25 In a reference 70 kg person, this amounts to 38 trillion bacteria, with a total mass of about 0.2 kg, the vast majority of which are found in the gut, particularly the colon.21,25
Gut microorganisms have evolved over thousands of years to form a remarkable symbiotic relationship with their hosts. In return for food via dietary intake, microbes provide a range of immune, digestive, and nutritional functions that are beneficial to the host.26,27 The microbiome and its consortium of genes may be thought of as an extension of the host’s genetic repertoire to form what some are now calling the ‘extended genotype’.28 Through modification of its host phenotype, this may mean that the microbiome even has the potential to modify human evolution.28
The microbiome is not static; it is a dynamic community that changes across the lifespan as individuals age.29,30 Considerable differences in the composition, abundance, and function of the gut microbiota have been noted between young and elderly individuals in clinical studies, often demonstrating a decrease in Bifidobacterium and Lactobacillus, and an increase in Enterobacteriaceae.30,31 These changes in the microbiome are associated with factors such as mucosal thinning, immune senescence, changes in diet and physical activity, medications, and health status that occur as individuals age.1,32 This phenomenon has led to the idea that age-related shifts in the gut microbiome may be associated with the predisposition of elderly people to certain diseases.31,33
Shifts in the microbiome may be associated with predisposition to certain diseases.1,31,33
The microbiome can be altered by numerous factors such as diet, genetics, environmental exposures, health status, and medications.1 Intestinal microorganisms also interact with the host through secreted toxins, by-products, and metabolites to modulate immune responses, endocrine secretion, metabolism, and neurotransmission.34 Microbiome changes may result in a shift towards an overabundance of bacteria associated with an ‘unhealthy’ or pathological state. This type of change in the microbiome is generally termed dysbiosis and has been associated with a range of metabolic, gastrointestinal, and neurological diseases including PD.1,20,30,31,35-37
A role for the gut microbiome in PD
Numerous studies have examined the gut microbiome of patients with PD and have found it to be altered compared with healthy controls.38-41 In a recent large-scale metagenomics analysis of the gut microbiome of 490 patients with PD, more than 30% of the microbial species, genes, and pathways tested demonstrated alterations compared with non-PD controls, depicting widespread dysbiosis.42 Attempts to identify the precise microorganisms associated with PD have produced inconsistent results. At the genus level, meta-analyses have shown that the relative abundance of anti-inflammatory and short chain fatty acid-producing bacteria (including Blautia, Coprococcus, Roseburia, Lachnospira, and Faecalibacterium) are reduced in PD patients compared to controls, whereas levels of Lactobacillus, Bifidobacterium, and Akkermansia are raised. Opportunistic pathogens and pro-inflammatory bacteria (including Alistipes, Escherichia, Bacteroides Corynebacterium, and Porphyromonas) are also enriched in patients with PD.34,36,43,44 It is still not clear if the observed changes in the microbiome of patients with PD are the initial occurrence that contributes to the development of the disease, or whether the changes emerge in response to PD pathology; both are a possibility.19
Interestingly, various gut disorders (including infections, dysbiosis, inflammation, and dysmotility), as well as dietary factors, have been shown to influence the response to PD medication.1 Microbial-directed therapeutics are showing some promise in the management of PD. Small and short-term pilot studies have shown improvements in motor and non-motor symptoms such as constipation, and levodopa bioavailability, with dietary interventions and supplementation with probiotics/prebiotics.1 Other potentially useful modalities being tested include faecal microbiota transplantation and small-molecule drugs, biologics, and metabolites acting on gut-related targets.1 Strategies targeting PD pathologies (such as alpha-synuclein aggregation) in the enteric nervous system are also in development and have recently demonstrated clinical trial success involving patients with PD.1,45,46 Ultimately, it is hoped that scientific advances in this field will translate to the responsible use of new diagnostic, prognostic, and therapeutic approaches that will improve the lives of patients living with PD.
Click here to access a collection of Microbiome and Parkinson’s Disease