Although motor symptoms of resting tremor, bradykinesia, rigidity, and postural instability are primary features of Parkinson’s disease (PD), many patients experience non-motor symptoms such as disorders of mood, cognition, sleep, and gastrointestinal or urinary function (Figure 1).1 It appears that certain non-motor symptoms can precede the development of motor symptoms by many years, and in some patients, they may remain the dominant features of the disease.2, 3
The nature and extent of non-motor symptoms vary markedly between patients and according to disease stage; and perhaps also according to aetiology, if we accept that PD is in fact not a single entity but an umbrella term covering many different biological subtypes.4 On any understanding of the disease, control of non-motor symptoms seems increasingly important to maintaining quality of life.5
Non-motor symptoms may affect the individual’s quality of life well before a formal diagnosis of PD is made and there is a significant correlation between the total number of non-motor symptoms and reduction in quality of life.5
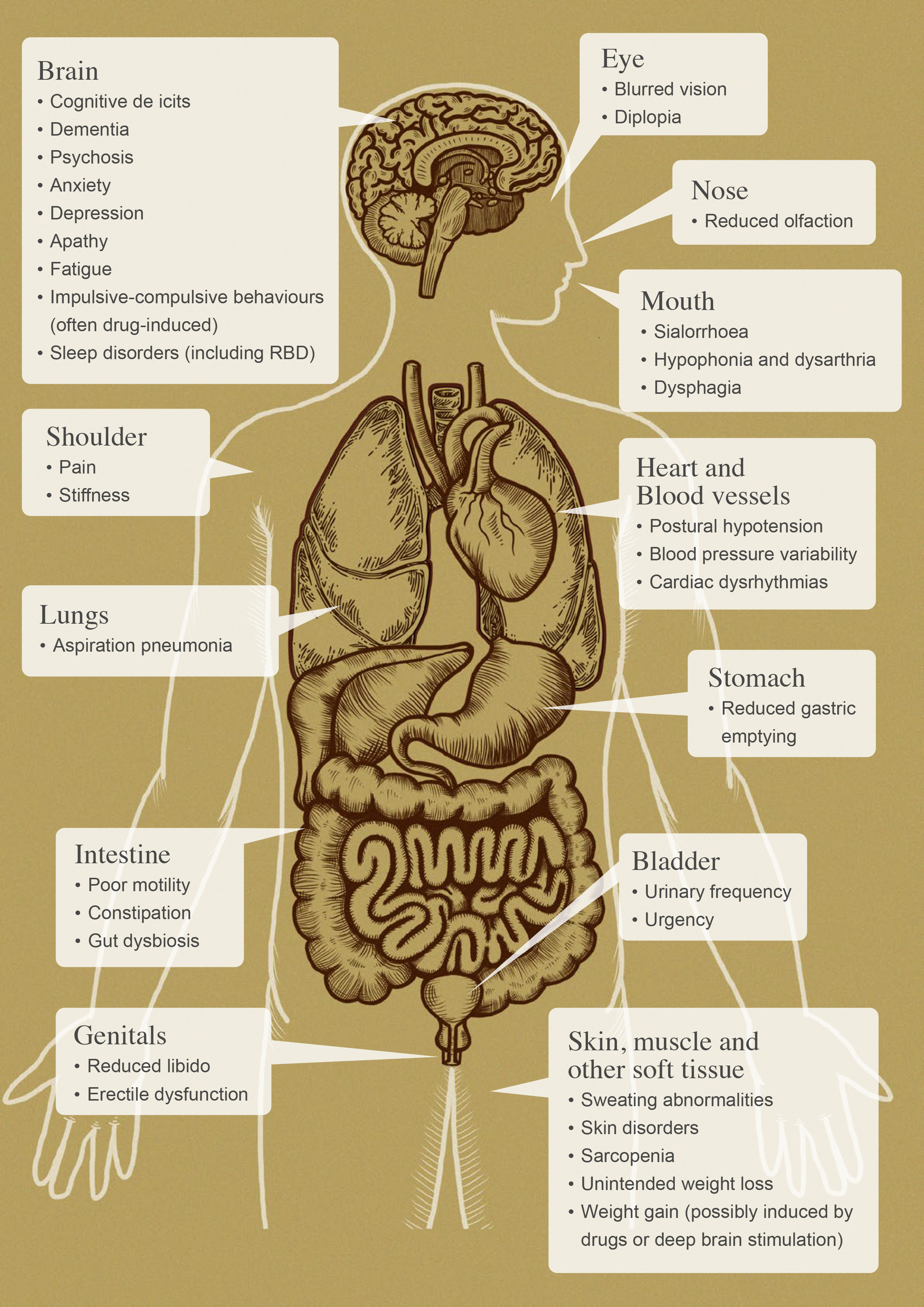
Figure 1. Potential non-motor features associated with Parkinson’s disease (adapted from Schapira et al 2017).1, 50, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64
Burden: the voice of patients and carers
In the FDA’s Patient-Focused Drug Development Initiative, patients made the following points:6
- Parkinson’s disease impacts all aspects of their lives, limiting ability to work, care for themselves and others, and to maintain relationships.
- When asked to identify up to three symptoms with the greatest impact on daily life, the highest number of responses related to core motor symptoms of slowed movement and tremor, followed by impaired balance and coordination, then by cognitive impairment, and disturbed sleep.
- Impaired balance and co-ordination were regarded as a major challenge, leading to falls and fear of falling.
- Fatigue and constipation were also highlighted as problems.
In an earlier study of patients’ perspectives, Marios Politis and colleagues from a group of London hospitals noted that patients and clinicians may differ in their views of what constitute the most troublesome symptoms.7 Among people who had had PD for six or more years, the greatest problem – in terms of quality of life – was fluctuating response to medication; this was followed by mood changes, drooling and sleep problems, and then by tremor. Problematic non-motor aspects of the disease such as pain and sleep, mood and bowel symptoms were also frequent in patients who had had PD for fewer than six years.7
“Parkinson’s disease is not just a disease of the brain, it is not just a disease of dopamine, and – above all – it is not just a disorder of movement.1”
Non-motor symptoms contribute to overall healthcare costs and have a profound impact on the quality of life of both patients and caregivers.5, 8, 9 Their improved management, including the development and use of non-dopaminergic therapies, has been identified as a major unmet medical need.9, 10 The range and potential severity of non-motor symptoms also argue strongly for the involvement of a multidisciplinary team and, in late-stage disease, for the integration of neurological and palliative care.9, 11
In the pre-motor phase of PD, a range of non-motor symptoms seems to be the norm, with gastrointestinal symptoms, olfactory impairment and rapid eye movement (REM) sleep behaviour disorder being particularly frequent.9,12 The possible value of a “prodromal signature” based on non-motor symptoms and the relationship of such symptoms to our understanding of the complex aetiology and staging of PD are considered in a companion article.
Interestingly, James Parkinson, in his seminal 1817 Essay on the Shaking Palsy, recognised that non-motor symptoms were also involved in the disease. “Sleep,” he wrote, “becomes much disturbed”. And the bowels, “which had been all along torpid, now, in most cases, demand stimulating medicines of very considerable power”. Although he could not explain how such a connection between brain and bowels might come about, Parkinson thought it possible that disorder of the gut could induce a “morbid action” and “derangement of structure” in the nervous system.13
Nature and prevalence of non-motor symptoms
The Sydney Multicenter Study, which followed 136 people newly diagnosed with PD, provides a valuable insight into the nature and prevalence of non-motor symptoms among long-term survivors.14 By twenty years, 100 had died. Of the 30 cohort members still alive to be included in the 20-year follow-up (mean age 74 years), 83% had dementia, 74% experienced hallucinations, and 71% had urinary incontinence. Falls had occurred in 87%, and 48% experienced symptomatic postural hypotension.14
One of the authors’ conclusions was that, in people with PD who survive sufficiently long, dementia is almost inevitable14 (although other authors subsequently reported somewhat lower rates – around 50% – of dementia on long-term follow-up).15 Another conclusion was that the diverse features of advanced PD go far beyond a lack of dopamine.14
In a prospective study of 113 people with PD (mean age 67; mean disease duration 5.7 years) attending two Montreal movement disorder clinics, Fereshtehnejad and colleagues found hallucinations at baseline in 7─26% of patients.16 The proportion depended on disease subtype, which was subsequently defined by cluster analysis of clinical features and rates of progression. Hyposmia was present in 84─95% of patients, and mild but multidomain cognitive impairment in 21─67%.16
Parkinson’s disease is a multi-system condition which, in addition to motor symptoms, involves four major domains of non-motor symptoms:
- neuropsychiatric (including psychosis, depression, apathy, impaired cognition and dementia)
- sleep-related
- autonomic (including gastrointestinal, urinary and sexual dysfunction; and orthostatic hypotension), and
- sensory (including pain and olfactory disturbance).
The prevalence of non-motor symptoms differs depending on the population studied, varying with factors such as age and duration of disease, but also with the definitions used. However, there is consensus that such problems are frequent and contribute substantially to the overall burden of PD. Table 1 contains some representative estimates of prevalence.
| Symptom | Prevalence | Reference |
| Delusions | 5─10% | Goldman et al. 201417 |
| Impulse control disorders | 14─46% | Weintraub et al 201018 Corvol et al. 201819 |
| Depression | 15% to >50% | Politis et al. 20107 Obeso et al. 20172 |
| Urinary dysfunction | 27─85% | Borghammer et al. 201720 |
| Orthostatic hypotension | 30% | Velseboer et al. 201121 Merola et al 201622 |
| Dementia | 30%, but more than 80% long term | Hely et al. 200814 Obeso et al. 20172 |
| Anxiety | 30─40% | Obeso et al. 20172 |
| Hallucinations | 40% | Fénelon et al. 200023 |
| Constipation | 40─50% | Borghammer et al. 201720 |
| REM sleep behaviour disorder | 50% | Rolinski et al 201424 |
| Psychosis | >50% | Goldman et al. 201417 |
| Impaired cognition* | Up to 67% | Fereshtehnejad et al. 201516 |
| Olfactory | Up to 90% | Obeso et al. 20172 Fereshtehnejad et al 201516 Doty et al 201225 |
*mild, multidomain
Table 1. Estimates of prevalence of non-motor symptoms in PD.
In addition to the non-motor symptoms that are intrinsic to PD, non-motor symptoms can be induced or worsened to a greater or lesser extent by the use of anti-parkinsonian drugs used to improve movements.26 For example, impulse control disorders and sleep attacks are strongly associated with the use of dopamine agonist therapy.26 There are also emerging data pointing to differences in non-motor symptoms profiles between different ethno-geographic populations.27, 28
Sleep disorder
The majority of people with idiopathic REM sleep behaviour disorder, a condition characterised by loss of normal REM sleep muscle atonia (allowing the acting out of dreams) will eventually be diagnosed with PD or a related synucleinopathy.29 St Louis and colleagues cite a range of 40─90% among longitudinal series of RBD patients followed for six years or longer.29
“Idiopathic REM sleep behaviour disorder looks like a very early manifestation of synucleinopathy 29”
As a prodromal feature, REM sleep behaviour disorder probably has the greatest predictive power of all the non-motor symptoms associated with PD. Also striking is the length of time by which the disorder can precede PD, which, in some cases, may even be diagnosed only several decades after the sleep problem becomes apparent.30
Estimating the prevalence of REM sleep behaviour disorder in established PD is made difficult by differences in definition. However, estimates suggest it is in the region of 25─30% in newly diagnosed patients and those with early disease.31
The view that REM sleep behaviour disorder is early evidence of a widespread underlying synucleinopathy is supported by its association with other markers of neurodegeneration such as cognitive impairment, constipation, orthostatic hypotension, and hyposmia.29
Early REM sleep behaviour disorder may have a place in subtyping PD using the ‘brain-first’ versus ‘body-first’ hypothesis in which REM sleep behaviour disorder has been proposed as a prodrome for the ‘body-first’ phenotype, although this concept remains contentious.32, 34
Neuropsychiatric symptoms
Associated with psychosis
In a classic paper based on detailed assessments of 216 people with PD, Gilles Fénelon and colleagues found that 40% had experienced hallucinations – visual in the majority of cases – in the previous three months.23 The authors suggested that this high prevalence could not be explained solely as a side effect of chronic dopaminergic treatment: hallucinations can occur in newly diagnosed patients; and cognitive impairment and disturbances in the sleep-wake cycle were identified as important risk factors.
In a review by Goldman and Holden, attention was drawn to adverse effects of both psychosis and dementia on the quality of life of PD sufferers and caregivers alike.17 The authors estimated that the prevalence of psychosis in PD is probably over 50%, and emphasised that psychosis and dementia are interlinked phenomena associated with increased need for nursing home placement and higher rates of morbidity and mortality.17 In a retrospective study of 445 patients who had died with a pathologically confirmed diagnosis of PD and a mean age of onset of 60.4 years and a mean age at death of 76.2 years, 50% had a history of visual hallucinations and/or minor psychotic symptoms.35 This provides support for the view that the lifetime prevalence of psychosis in PD is indeed around 50%.
Genetic and other factors related to the risk of early PD-related psychosis are being identified36, 37; and recent evidence for the involvement of widespread cortical thinning and hippocampal volume loss is intriguing.38
Dementia and cognitive impairment
Progressive cognitive impairment affecting a wide range of domains is frequent and has been described as one of the most significant non-motor symptoms associated with PD.2 Cognitive deficits in domains such as memory, attention, and executive function, have been reported even in prodromal PD,39 and up to 80% of patients with a long history of the disease are thought to develop dementia.17 It has been estimated that 10% of people with PD develop dementia each year.17
“Cognitive and psychiatric issues, particularly dementia, psychosis, apathy, and depression in patients with PD can place a considerable burden on the caregiver41, 42”
The principal pathology is thought to be the diffuse presence of cortical Lewy bodies.2 The range of neurotransmitters involved in PD-associated cognitive impairment includes acetylcholine and norepinephrine, as well as dopamine. Glucocerebrosidase (GBA) gene polymorphisms and mutations have been linked to substantially increased risk of PD dementia.40
Anxiety and depression
Anxiety and depressive disorders are common neuropsychiatric complications of PD. The difference in these disorders between PD and non-PD patients is not clearly defined. Depression in PD can be major, minor, or dysthymic, and anxiety can present with panic attacks, phobias, or generalised anxiety disorder.43 Both disorders can be episodic or non-episodic and may be associated with OFF periods, with or without motor features.43 Depression and anxiety are likely due to a complex interplay between neurobiological factors and the psychological consequences of having a chronic, degenerative disease.44 In the case of depression, neurobiological factors have been reported to include abnormalities of basotemporal limbic circuitry and a range of neurotransmitters such as norepinephrine, serotonin and dopamine.44
Autonomic dysfunction
Lewy bodies are frequently found in the autonomic nervous system of people who die with PD, and autonomic dysfunction in various forms is a common part of PD symptomatology.45 Autonomic problems can include erectile and urinary dysfunction, although these conditions are so closely related to age that it is not straightforward to disentangle the role of PD.46 However, the association of PD with gastrointestinal dysfunction – aspects of which may precede the onset of motor symptoms by many years – is both clear and intriguing.47, 48
Gastrointestinal dysfunction
Radiotracer and imaging studies have shown extensive pathology of the sympathetic and parasympathetic nervous systems in people with PD.20 Disordered pharyngeal and oesophageal motility is frequently evident, along with delayed gastric emptying and colonic transit.20
Chaudhuri and colleagues cited an estimate that more than 70% of PD patients have gastrointestinal disorders.9 These include gastric dysmotility and small intestinal bacterial overgrowth. In addition to the associated morbidity, these conditions may have implications for the impaired absorption of oral drugs, notably levodopa.47, 48
A history of constipation is associated with an increased risk of PD, preceding diagnosis significantly more frequently in those who develop the disease than in matched controls.43 Notably, the presence and severity of constipation at baseline has recently been shown to predict worse clinical outcome in the medium to longer term, including faster progression to dementia.48, 49
Orthostatic hypotension
The autonomic dysfunction associated with synucleinopathies can prevent sufficient compensatory release of norepinephrine from sympathetic nerves, leading to hypotension on standing.50
A meta-analysis of 25 studies published up to 2009 reported a pooled prevalence estimate of 30% of orthostatic hypotension in patients with PD.21 A comparable prevalence was reported in a 2016 study in which similar impairments in functional ability were found in patients with symptomatic and asymptomatic orthostatic hypotension, suggesting that affected patients may not always present with overt symptoms.22
Lim and Lang considered whether orthostatic hypotension is intrinsic to PD or caused predominantly by PD medication treatment.26 They concluded that evidence of reflexive cardiovagal failure, sympathetic neurocirculatory failure and extracardiac noradrenergic denervation in a substantial proportion of untreated PD patients suggests it is substantially due to the underlying disease process.26
Orthostatic hypotension has significant effects on quality of life since falls and the fear of falls limit social and physical activity. It has also been associated with other significant issues such as cognitive impairment.51, 52 In recognition of the importance of this problem, the American Autonomic Society and the National Parkinson Foundation have issued joint recommendations on screening for orthostatic hypotension and its management.50
Olfactory dysfunction
Olfactory dysfunction is another non-motor symptom closely associated with PD and predictive of its development. Doty estimated that around 90% of people with early PD experience olfactory dysfunction.25 This symptom is shared with many Alzheimer’s disease patients, but is not as apparent in multiple system atrophy and progressive supranuclear palsy, making loss of smell potentially valuable as a means of distinguishing between parkinsonian syndromes. Like several other non-motor symptoms such as RBD and constipation, hyposmia is also recognised to occur early as a prodromal non-motor feature of PD.25
Criteria for diagnosing PD by the Movement Disorder Society
While the latest Movement Disorder Society Clinical Diagnostic Criteria retain bradykinesia plus rest tremor or rigidity as the core features of parkinsonism, they give new emphasis to non-motor aspects of the disease.53 This is intended to better reflect current understanding and diagnostic practice in expert centres.
Once parkinsonism has been documented, the judgement that PD is the cause relies on three categories of diagnostic features:
- absolute exclusion criteria, which rule out PD (dementia has now been removed as an exclusion criterion for PD, even if it is the first presenting symptom)
- red flags, which must be counterbalanced by additional supportive criteria to allow diagnosis of PD, and
- supportive criteria i.e., positive features that increase confidence in the PD diagnosis.
Loss of olfaction is one such supportive feature. The other side of the coin is that the absence of any of the common non-motor features (despite at least five years’ disease duration) is regarded as a red flag. The relevant non-motor symptoms include REM sleep behavior disorder, constipation, symptomatic orthostasis, and psychiatric dysfunction (depression, anxiety, or hallucinations).53
“Up to 90% of people with early PD have loss or reduced sense of smell2”
Non-motor features were regarded as especially important in the development of the MDS’s companion set of criteria for prodromal PD.54 These criteria are intended at least initially for use in a research context and acknowledge that early PD pathology and related symptoms involve the peripheral nervous system and non-dopaminergic brain structures. The likelihood of prodromal PD is calculated based on age, environmental and genetic risk factors, biomarkers (e.g., dopaminergic imaging) and prodromal symptoms and signs such as REM sleep behaviour disorder, constipation, and hyposmia.54